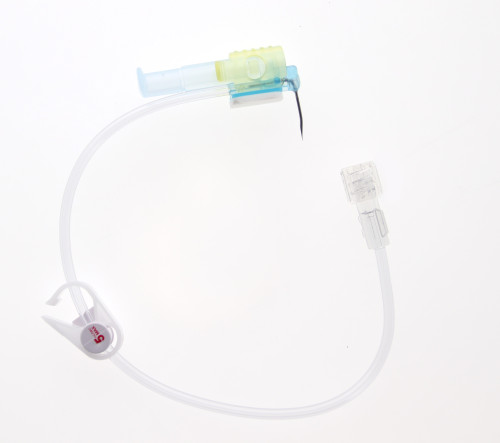
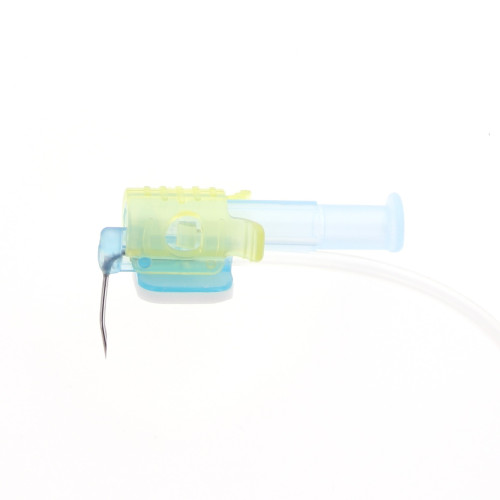
Safety Huber needle
PPS CT
Beskrivning
The PPS® CT is a curved safety Huber needle supplied with one connecting line ideal for accessing pressure injectable ports. This device can be… Läs mer
Indikation
PPS® CT safety Huber needles are indicated for:
•• Administration or withdrawal of fluids through implantable ports.
•• Pressure injection of contrast media into the central venous system only with an implantable infusion port that is also indicated for pressure injections.
The maximum recommended infusion rate at 11.8 cPs is:
–– 5 mL/sec for a 19G needle. (1.1 mm)
–– 5 mL/sec for a 20G needle. (0.9 mm)
–– 2 mL/sec for a 22G needle. (0.7 mm)
PPS® CT safety Huber needles are indicated to prevent blood-borne pathogen exposures caused by accidental needle punctures (accidental blood exposure). They do not protect against other routes of blood-borne pathogen transmission.
Produktens höjdpunkter
Compatible with high-pressure injections up to 300 psi:
PPS® CT is compatible with MRI procedures and contrast media injection during CT examinations. The maximum perfusion rate is indicated on the needle clamp.
Single-handed positive pressure:
PPS® CT's design allows to perform positive pressure during needle removal with one hand. This helps prevent catheter occlusions (1) (2) (3).
Prevention of Needle Stick Injuries (NSI):
PPS® CT is a safe Huber needle offering protection against the risks of NSI (4). During disposal, there is no contact between the body and the needle tip.
From paediatric to adults : PPS® CT is part of a wide range of Huber needles to suit all patient morphologies:
- 3 gauges (22G, 20G and 19G)
- 6 lengths (15, 17, 20, 25, 30, 35 mm).
PPS® CT is available with or without a Y site.
(1) Gorski LA, Hadaway L, Hagle ME, Broadhurst D, Clare S, Kleidon T, Meyer BM, Nickel B, Rowley S, Sharpe E, Alexander
M. Infusion Therapy Standards of Practice, 8th Edition. J Infus Nurs. 2021 Jan-Feb 01;44(1S Suppl 1):S1-S224. doi: 10.1097/
NAN.0000000000000396. PMID: 33394637.
(2) Lapalu J, Losser MR, Albert O, Levert A, Villiers S, Faure P, Douard MC. Totally implantable port management: impact of
positive pressure during needle withdrawal on catheter tip occlusion (an experimental study). J Vasc Access. 2010 Jan-
Mar;11(1):46-51. doi: 10.1177/112972981001100110. PMID: 20175068.
(3) Levert H, Albert O, Barret E, Villiers S, Douard MC, A randomized experimental comparison of two safety Huber
needles (HN) allowing manual or automatic positive pressure during needle removal: effect on the distal catheter
reflux, poster, WoCoVA, 2014.
(4) Survey of the occurrence circumstances of Accidental Blood Exposure due to punctures with safety materials, GERES – AFSSAPS Collaboration,
G. Pellissier, 18th Annual GERES conference, 2008.
The Huber needle PPS® CT is a sterile medical device of class IIa.
Certification established by GMED, notified body n°0459.
They are indicated for the administration into or withdrawal of fluids from implanted ports.
Read the instructions for use carefully.
Device manufactured by PEROUSE MEDICAL and distributed by VYGON.
Beskrivning
The PPS® CT is a curved safety Huber needle supplied with one connecting line ideal for accessing pressure injectable ports. This device can be used for pressure injection of contrast media (up to 300 psi / 2068 kPa maximum) during CT scan (Computerized Tomography) or MRI (Magnetic Resonance Imaging) exams, and is MR Conditional (see details in parts V of this IFU).The PPS® CT curved safety Huber needle is available:
•• in several lengths and diameters
•• with or without Y-site
PPS® CT codes 80xxxx are without a Y site.
PPS® CT codes 81xxxx are with a Y site.
It is supplied with:
•• Luer-Lock connectors on which closed protective caps are screwed (end of the main line and of the Y site) which must not be used as sealing caps.
•• and one clamp (2 clamps for references including an Y-site) indicating the maximum flow rate of the PPS® CT.
The PPS® CT is not composed of natural latex (raw-materials and packaging) with DEHP-free tubing.
Referenser och funktioner
| Needle | Förpackning | ||||||
|---|---|---|---|---|---|---|---|
| Favourites | Kod | Diameter G | Ext. Ø mm | Length mm | Priming volume ml | Antal/Avd.förpackning | Antal/Trp.förpackning |
| 801507 | 22 | 0.7 | 15 | 0.50 | 12 | 360 | |
| 801509 | 20 | 0.9 | 15 | 0.50 | 12 | 360 | |
| 801511 | 19 | 1.1 | 15 | 0.50 | 12 | 360 | |
| 801707 | 22 | 0.7 | 17 | 0.50 | 12 | 360 | |
| 801709 | 20 | 0.9 | 17 | 0.50 | 12 | 360 | |
| 801711 | 19 | 1.1 | 17 | 0.50 | 12 | 360 | |
| 802007 | 22 | 0.7 | 20 | 0.50 | 12 | 360 | |
| 802009 | 20 | 0.9 | 20 | 0.50 | 12 | 360 | |
| 802011 | 19 | 1.1 | 20 | 0.50 | 12 | 360 | |
| 802507 | 22 | 0.7 | 25 | 0.50 | 12 | 360 | |
| 802509 | 20 | 0.9 | 25 | 0.50 | 12 | 360 | |
| 802511 | 19 | 1.1 | 25 | 0.50 | 12 | 360 | |
| 803007 | 22 | 0.7 | 30 | 0.50 | 12 | 360 | |
| 803009 | 20 | 0.9 | 30 | 0.50 | 12 | 360 | |
| 803011 | 19 | 1.1 | 30 | 0.50 | 12 | 360 | |
| 803507 | 22 | 0.7 | 35 | 0.50 | 12 | 360 | |
| 803509 | 20 | 0.9 | 35 | 0.50 | 12 | 360 | |
| 803511 | 19 | 1.1 | 35 | 0.50 | 12 | 360 | |
| 811507 | 22 | 0.7 | 15 | 0.70 | 12 | 360 | |
| 811509 | 20 | 0.9 | 15 | 0.70 | 12 | 360 | |
| 811511 | 19 | 1.1 | 15 | 0.70 | 12 | 360 | |
| 811707 | 22 | 0.7 | 17 | 0.70 | 12 | 360 | |
| 811709 | 20 | 0.9 | 17 | 0.70 | 12 | 360 | |
| 811711 | 19 | 1.1 | 17 | 0.70 | 12 | 360 | |
| 812007 | 22 | 0.7 | 20 | 0.70 | 12 | 360 | |
| 812009 | 20 | 0.9 | 20 | 0.70 | 12 | 360 | |
| 812011 | 19 | 1.1 | 20 | 0.70 | 12 | 360 | |
| 812507 | 22 | 0.7 | 25 | 0.70 | 12 | 360 | |
| 812509 | 20 | 0.9 | 25 | 0.70 | 12 | 360 | |
| 812511 | 19 | 1.1 | 25 | 0.70 | 12 | 360 | |
| 813007 | 22 | 0.7 | 30 | 0.70 | 12 | 360 | |
| 813009 | 20 | 0.9 | 30 | 0.70 | 12 | 360 | |
| 813011 | 19 | 1.1 | 30 | 0.70 | 12 | 360 | |
| 813507 | 22 | 0.7 | 35 | 0.70 | 12 | 360 | |
| 813509 | 20 | 0.9 | 35 | 0.70 | 12 | 360 | |
| 813511 | 19 | 1.1 | 35 | 0.70 | 12 | 360 | |
Ytterligare information
-
Innehåller latex Nej
-
Innehåller animaliska produkter Nej
-
Pyrogenfri Nej
Dokumentation
-
Brochure_PPS_CT
-
Brochure Vascular Access Range
-
Instruktioner för användning